Our offer
Products
Find biomarker signatures and identify therapeutic avenues.
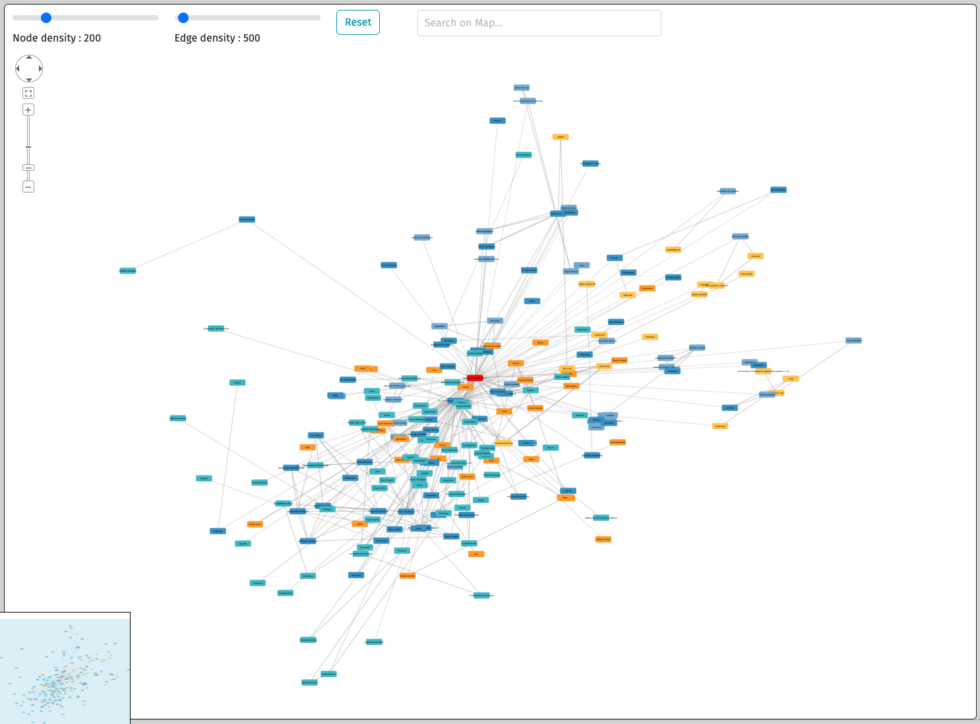
Interactive numerical disease models library
Explore diseases with our maps.
Browse the signs and symptoms of the disease as well as the cells,
proteins, biological and medical processes involved.
Smart Disease Analyzer
Identify biomarkers and compare diseases
Navigate your way through the following tools.
Data-Driven Disease Insight
You want custom support?
We help pre-clinical R&D project managers by integrating physiological dimension
in the big picture.
Testimonials
"Strong prospective insight from identification of mechanism of action in Breast cancer progression"

E. B.
Research Director CNRS, France"Fresher and more powerfull than a chatGPT for health, far more advanced than the regular offer"

Undisclosed
Stakeholder, Mayo Clinic, USA"You are one of the very few that are working on something is of outmost importance: causality"

E.G.
Senior director, Artificial Intelligence Institute, France"Really cool work"

L.LD.
Senior Business Developper and serial entrepreneur, Serie B and IPO companies, USA"Really interesting approaches, totally in phase"

M.C.
MD, Professor and Director of Hospital and associate partner, International Clinical cluster"Happy to have found you"

B. B.
Pharm.D., Professor, France"Answering totally to an unmet need in medicine"

F.B.
MD, Professor, University Hospital, France"Super interesting and exciting"

MB. M.
MD, Professor, University Hospital, France"Intersting insights from complex experimental data"

J.P.
MD and CSO, Serie B Biotech company, Belgium"Exactly what we need"

A.BT.
CEO, Medtech company, France"We have to work together"

T.S.
CEO, Medtech company, France"Really powerfull for clinical trials and pivots"

D.P.
Business developper, Roche Diagnostics, France"Very interesting value proposition and offer, you should definitely push it forward"

Undisclosed
Stakeholder, European Space Agency"Very happy to work together"

P.K.
Professor, MIT fellow and Partner in Medtech multinational Serie B company"Very deep and exhaustive research information for our consortium"

O.S.
Senior Partner, PWC, Switzerland"Thank you a lot for your help, you saved us so much time!"

I.H.
Partner, PWC, Swirtzerland
Reckonect Diagnostic & Therapeutic Science Hub
Reckonect - Frequently Asked Questions - FAQ
Reckonect SAS is a company rooted in deeptech applied to biology. We combine AI-based information retrieval models and biomedical expertise to map disease progression from 3 perspectives: scale, causality and context of pathological development.
We are distinct from alternatives, noteworthy because we rely on avatars of pathologies and we can integrate heterogenous data (eg: multi-omics, semantic). See more at reckonect.com.
Drug screening for the identification of lead and mechanism of action, is expensive
(several thousands of euros), long (3-5 years) with a high failure rate as only 4% will yield a licensed
drug.
Reckonect’s AI based in silico drug screening for preclinical studies, drastically decreases both
the duration
and expenses of this step.
We offer several solutions for in silico drug screening, de novo
positioning,
indication repositioning or even identification of mechanism of action.
Ideally positioned upstream
of the in
vitro testing you designed; you alleviate the risk of failing the in-vitro tests and save both time and
money by at
least a factor of 100 folds.
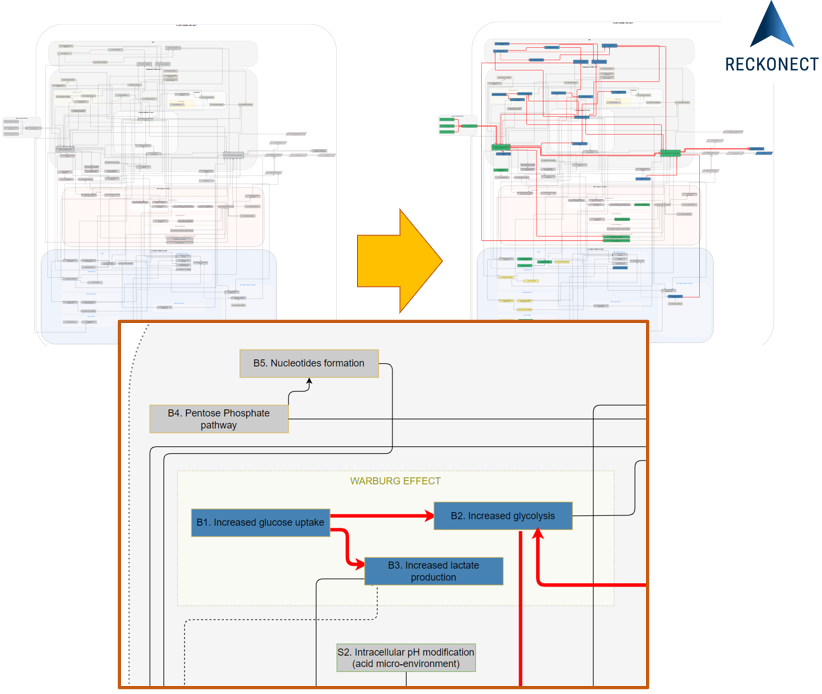
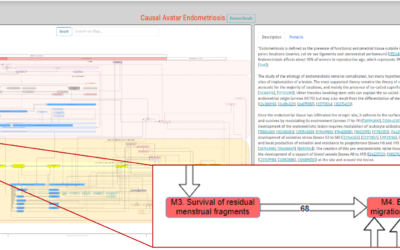
A mechanistic disease progression model is a representation of all accessible
relevant knowledge and validated experimental, preclinical, clinical data or even real-world evidence.
It is
built upon the understanding of physiology in depth, especially on the expertise of the hierarchy of
biological
events.
The most promising model of disease progression are avatars and digital twins in healthcare.
Avatars as mechanistic disease progression models, is a game changer in diagnostics
and therapeutic R&D.
They are known to drastically compress both time and expenses of basic,
translational
and clinical research in diagnostics and therapeutics discovery.
They are especially relevant on the
following
tasks: the identification of biomarkers and drivers of disease progression, the unravelling of the
synergies or
antagonisms of comorbidities or genetic background on a given disease progression.
Mechanistic
disease
progression avatars rationalize very early the R&D process with the complex physiological knowledge.
They
are currently impacting profoundly therapeutic discovery as they to in silico drug screening, de novo
indication
positioning, and indication repositioning.
Disease is associated with a prototypical signature, aka several non-physiological
alterations in the behavior of biological components (e.g. genes, lipids, proteins, metabolites,
signaling networks,
cell types, organs).
More precisely, this signature of biomarkers and drivers, is composed of
distinct types
and identities of components (e.g. a given cellular ecosystem for which several signaling are altered)
and is
specific of associated conditions such as comorbidities.
Although analysis of omics data by data scientists is still the main strategy to
perform the discovery of biomarkers and drivers, it suffers from several caveats that mechanistic models
resolve.
Nowadays, mechanistic models such as avatars of disease progression, are combined with data science
tools.
Mechanistic models are a kind of avatars that is now combined with data science to offer the only
way of
integration of both biological cascades and physiological context, as well as the various organism
levels
(molecules, cell types, organs).
This dual strategy is proven to be extremely powerful as it
facilitates
empirical data integration (e.g. omics, imaging, semantics) and therefore impacts directly on candidate
discovery,
qualification, verification, validation and optimization.
Disease progression encompasses several levels of organization: molecular, cellular
and tissular. When it comes to understand and target disease progression, one has to carefully consider
the
existence of comorbidities or existing conditions.
In such cases, the classical statistical data
science on
omics or imaging data, remains too reductionist to take into account comorbidities.
On the contrary,
Reckonect
can easily build and combined mechanistic disease progression models of several conditions to identify
their
potential synergies or antagonisms, using and empowering datascience workflows.